| RESEARCH LETTER |
![]()
![]()
LOW-LEVEL ELECTRIC CURRENT AND CANCER --
A PROMISING, BUT LANGUISHING NON-TOXIC CANCER THERAPY
JayKulsh, MS#
![]()
INTRODUCTION -- SCIENTIFIC BASIS OF GEIPE CANCER THERAPY
At biochemical level, all cancers are alike. In order for a cell to
divide in any organ, its DNA strand must be replicated. Of the various
enzymes involved in the process of DNA synthesis, none is more critical
than ribonucleotide reductase (RnR), which provides the building
blocks -- four bases -- of DNA, by reducing the corresponding, abundantly
available, bases of RNA. The activity of this RnR enzyme is most
closely linked to malignant transformation and tumor cell
proliferation.1
COMPELLING EXPERIMENTAL EVIDENCE
Long before the mechanism was proposed on how low-level electric
current might stop cancerous growth, about 10 cancer electrotherapy
studies had been reported in the scientific literature. The results of
all these studies are consistent with the mechanism involving
destruction of the free-radicalcontaining active site of the enzyme
RnR.
|
less than 3 V, one hour per day for five days.6 It should be noted that poor results were obtained in a few studies that employed higher voltages, since at more than 4 V, electrochemistry takes place, resulting in toxic byproducts and leaving fewer electrons to quench the free radical. EFFECTIVENESS IN HUMAN PATIENTS
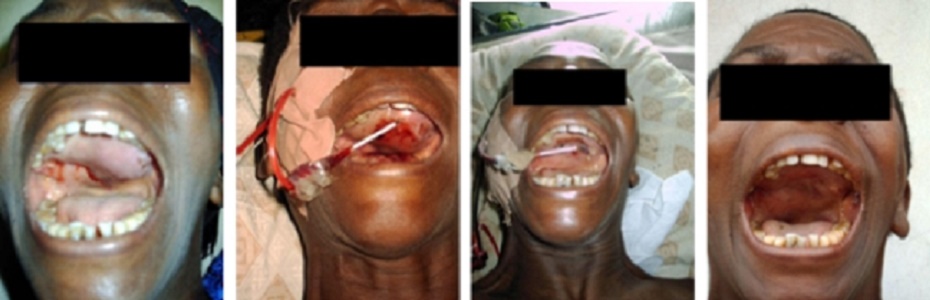
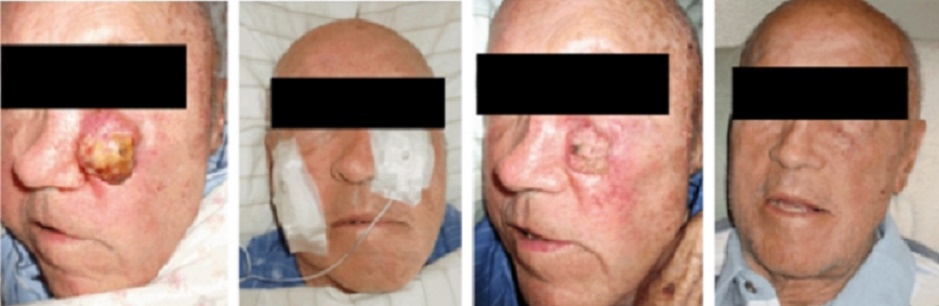
Over the years, the author has built various GEIPE devices, constantly
improving them and has treated a few patients whose cancer no longer
responded to conventional treat-ments or who were averse to conventional
toxic therapies.
UNIQUE DIFFICULTIES IN ESTABLISHING GEIPE THERAPY
More than 15 years ago, the MD Anderson Cancer Center, Houston and the
National Cancer Institute of USA acknowledged the validity of this
approach to treat cancer in correspondence with the author (personal
communication). However, no effort has been made by any institution to
explore and standardize this treatment, as the process would present
unique challenges.
|
![]()
ISSN 1550-8307/$36.00 http://dx.doi.org/10.1016/j.explore.2013.10.004